OpenStax College, OpenStax College
Chapter 9. Molecular Biology

Introduction*
The three letters “DNA” have now become associated with crime solving, paternity testing, human identification, and genetic testing. DNA can be retrieved from hair, blood, or saliva. With the exception of identical twins, each person’s DNA is unique and it is possible to detect differences between human beings on the basis of their unique DNA sequence.
DNA analysis has many practical applications beyond forensics and paternity testing. DNA testing is used for tracing genealogy and identifying pathogens. In the medical field, DNA is used in diagnostics, new vaccine development, and cancer therapy. It is now possible to determine predisposition to many diseases by analyzing genes.
DNA is the genetic material passed from parent to offspring for all life on Earth. The technology of molecular genetics developed in the last half century has enabled us to see deep into the history of life to deduce the relationships between living things in ways never thought possible. It also allows us to understand the workings of evolution in populations of organisms. Over a thousand species have had their entire genome sequenced, and there have been thousands of individual human genome sequences completed. These sequences will allow us to understand human disease and the relationship of humans to the rest of the tree of life. Finally, molecular genetics techniques have revolutionized plant and animal breeding for human agricultural needs. All of these advances in biotechnology depended on basic research leading to the discovery of the structure of DNA in 1953, and the research since then that has uncovered the details of DNA replication and the complex process leading to the expression of DNA in the form of proteins in the cell.
9.1. The Structure of DNA*
By the end of this section, you will be able to:
- Describe the structure of DNA
- Describe how eukaryotic and prokaryotic DNA is arranged in the cell
In the 1950s, Francis Crick and James Watson worked together at the University of Cambridge, England, to determine the structure of DNA. Other scientists, such as Linus Pauling and Maurice Wilkins, were also actively exploring this field. Pauling had discovered the secondary structure of proteins using X-ray crystallography. X-ray crystallography is a method for investigating molecular structure by observing the patterns formed by X-rays shot through a crystal of the substance. The patterns give important information about the structure of the molecule of interest. In Wilkins’ lab, researcher Rosalind Franklin was using X-ray crystallography to understand the structure of DNA. Watson and Crick were able to piece together the puzzle of the DNA molecule using Franklin’s data (Figure 9.2). Watson and Crick also had key pieces of information available from other researchers such as Chargaff’s rules. Chargaff had shown that of the four kinds of monomers (nucleotides) present in a DNA molecule, two types were always present in equal amounts and the remaining two types were also always present in equal amounts. This meant they were always paired in some way. In 1962, James Watson, Francis Crick, and Maurice Wilkins were awarded the Nobel Prize in Medicine for their work in determining the structure of DNA.

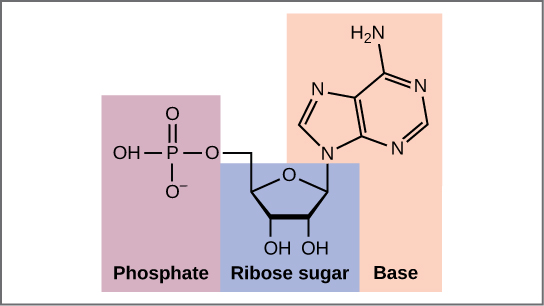
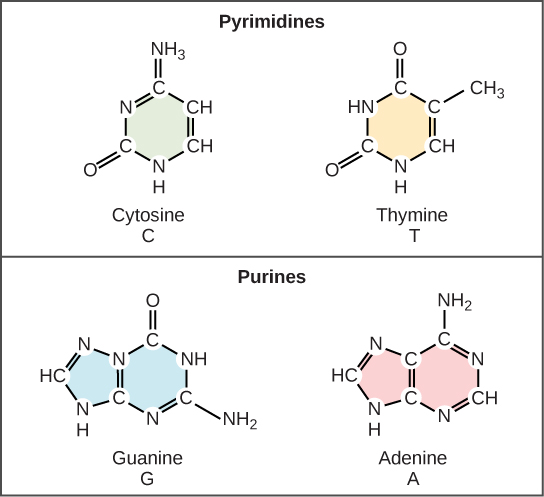
Now let’s consider the structure of the two types of nucleic acids, deoxyribonucleic acid (DNA) and ribonucleic acid (RNA). The building blocks of DNA are nucleotides, which are made up of three parts: a deoxyribose (5-carbon sugar), a phosphate group, and a nitrogenous base (Figure 9.3). There are four types of nitrogenous bases in DNA. Adenine (A) and guanine (G) are double-ringed purines, and cytosine (C) and thymine (T) are smaller, single-ringed pyrimidines. The nucleotide is named according to the nitrogenous base it contains.
(a)
|
(b)
|
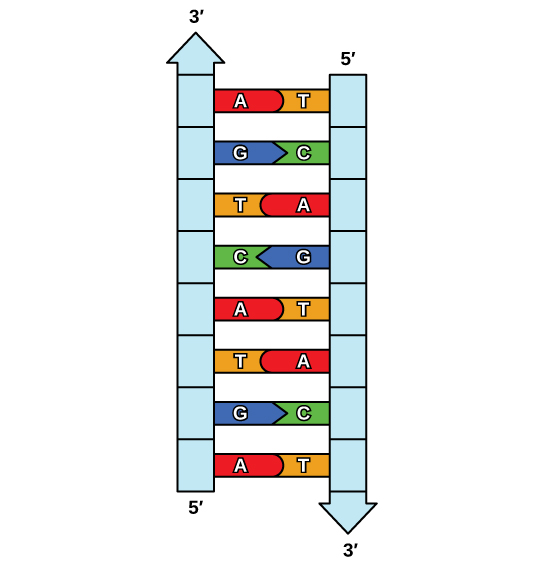
The phosphate group of one nucleotide bonds covalently with the sugar molecule of the next nucleotide, and so on, forming a long polymer of nucleotide monomers. The sugar–phosphate groups line up in a “backbone” for each single strand of DNA, and the nucleotide bases stick out from this backbone. The carbon atoms of the five-carbon sugar are numbered clockwise from the oxygen as 1′, 2′, 3′, 4′, and 5′ (1′ is read as “one prime”). The phosphate group is attached to the 5′ carbon of one nucleotide and the 3′ carbon of the next nucleotide. In its natural state, each DNA molecule is actually composed of two single strands held together along their length with hydrogen bonds between the bases.
Watson and Crick proposed that the DNA is made up of two strands that are twisted around each other to form a right-handed helix, called a double helix. Base-pairing takes place between a purine and pyrimidine: namely, A pairs with T, and G pairs with C. In other words, adenine and thymine are complementary base pairs, and cytosine and guanine are also complementary base pairs. This is the basis for Chargaff’s rule; because of their complementarity, there is as much adenine as thymine in a DNA molecule and as much guanine as cytosine. Adenine and thymine are connected by two hydrogen bonds, and cytosine and guanine are connected by three hydrogen bonds. The two strands are anti-parallel in nature; that is, one strand will have the 3′ carbon of the sugar in the “upward” position, whereas the other strand will have the 5′ carbon in the upward position. The diameter of the DNA double helix is uniform throughout because a purine (two rings) always pairs with a pyrimidine (one ring) and their combined lengths are always equal. (Figure 9.4).
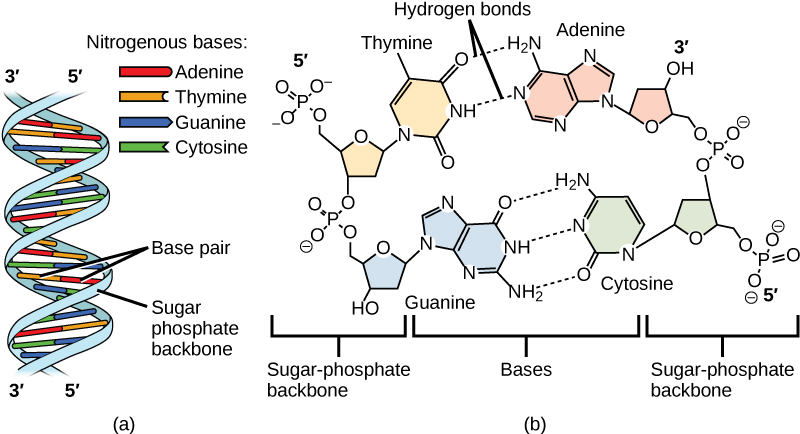
The Structure of RNA
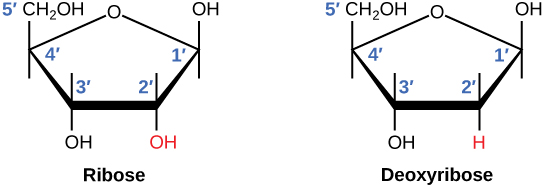
There is a second nucleic acid in all cells called ribonucleic acid, or RNA. Like DNA, RNA is a polymer of nucleotides. Each of the nucleotides in RNA is made up of a nitrogenous base, a five-carbon sugar, and a phosphate group. In the case of RNA, the five-carbon sugar is ribose, not deoxyribose. Ribose has a hydroxyl group at the 2′ carbon, unlike deoxyribose, which has only a hydrogen atom (Figure 9.5).
RNA nucleotides contain the nitrogenous bases adenine, cytosine, and guanine. However, they do not contain thymine, which is instead replaced by uracil, symbolized by a “U.” RNA exists as a single-stranded molecule rather than a double-stranded helix. Molecular biologists have named several kinds of RNA on the basis of their function. These include messenger RNA (mRNA), transfer RNA (tRNA), and ribosomal RNA (rRNA)—molecules that are involved in the production of proteins from the DNA code.
How DNA Is Arranged in the Cell
DNA is a working molecule; it must be replicated when a cell is ready to divide, and it must be “read” to produce the molecules, such as proteins, to carry out the functions of the cell. For this reason, the DNA is protected and packaged in very specific ways. In addition, DNA molecules can be very long. Stretched end-to-end, the DNA molecules in a single human cell would come to a length of about 2 meters. Thus, the DNA for a cell must be packaged in a very ordered way to fit and function within a structure (the cell) that is not visible to the naked eye. The chromosomes of prokaryotes are much simpler than those of eukaryotes in many of their features (Figure 9.6). Most prokaryotes contain a single, circular chromosome that is found in an area in the cytoplasm called the nucleoid.
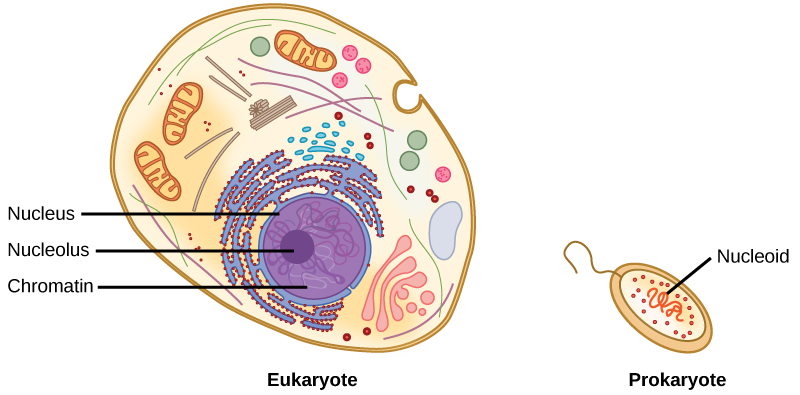
The size of the genome in one of the most well-studied prokaryotes, Escherichia coli, is 4.6 million base pairs, which would extend a distance of about 1.6 mm if stretched out. So how does this fit inside a small bacterial cell? The DNA is twisted beyond the double helix in what is known as supercoiling. Some proteins are known to be involved in the supercoiling; other proteins and enzymes help in maintaining the supercoiled structure.
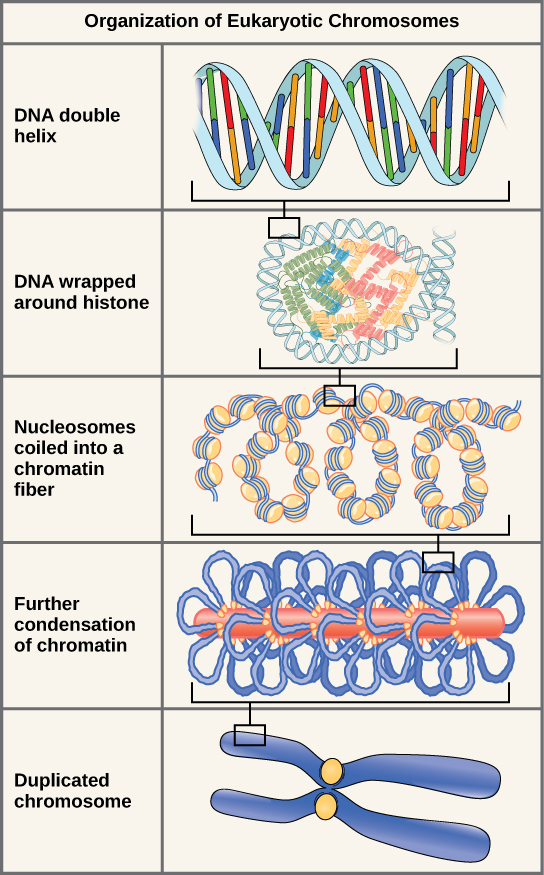
Eukaryotes, whose chromosomes each consist of a linear DNA molecule, employ a different type of packing strategy to fit their DNA inside the nucleus (Figure 9.7). At the most basic level, DNA is wrapped around proteins known as histones to form structures called nucleosomes. The DNA is wrapped tightly around the histone core. This nucleosome is linked to the next one by a short strand of DNA that is free of histones. This is also known as the “beads on a string” structure; the nucleosomes are the “beads” and the short lengths of DNA between them are the “string.” The nucleosomes, with their DNA coiled around them, stack compactly onto each other to form a 30-nm–wide fiber. This fiber is further coiled into a thicker and more compact structure. At the metaphase stage of mitosis, when the chromosomes are lined up in the center of the cell, the chromosomes are at their most compacted. They are approximately 700 nm in width, and are found in association with scaffold proteins.
In interphase, the phase of the cell cycle between mitoses at which the chromosomes are decondensed, eukaryotic chromosomes have two distinct regions that can be distinguished by staining. There is a tightly packaged region that stains darkly, and a less dense region. The darkly staining regions usually contain genes that are not active, and are found in the regions of the centromere and telomeres. The lightly staining regions usually contain genes that are active, with DNA packaged around nucleosomes but not further compacted.

Watch this animation of DNA packaging.
9.2. DNA Replication*
By the end of this section, you will be able to:
- Explain the process of DNA replication
- Explain the importance of telomerase to DNA replication
- Describe mechanisms of DNA repair
When a cell divides, it is important that each daughter cell receives an identical copy of the DNA. This is accomplished by the process of DNA replication. The replication of DNA occurs during the synthesis phase, or S phase, of the cell cycle, before the cell enters mitosis or meiosis.
The elucidation of the structure of the double helix provided a hint as to how DNA is copied. Recall that adenine nucleotides pair with thymine nucleotides, and cytosine with guanine. This means that the two strands are complementary to each other. For example, a strand of DNA with a nucleotide sequence of AGTCATGA will have a complementary strand with the sequence TCAGTACT (Figure 9.8).
Because of the complementarity of the two strands, having one strand means that it is possible to recreate the other strand. This model for replication suggests that the two strands of the double helix separate during replication, and each strand serves as a template from which the new complementary strand is copied (Figure 9.9).
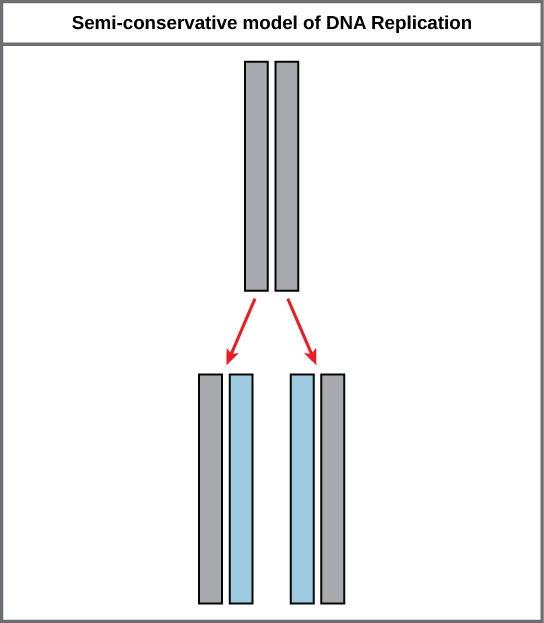
During DNA replication, each of the two strands that make up the double helix serves as a template from which new strands are copied. The new strand will be complementary to the parental or “old” strand. Each new double strand consists of one parental strand and one new daughter strand. This is known as semiconservative replication. When two DNA copies are formed, they have an identical sequence of nucleotide bases and are divided equally into two daughter cells.
DNA Replication in Eukaryotes
Because eukaryotic genomes are very complex, DNA replication is a very complicated process that involves several enzymes and other proteins. It occurs in three main stages: initiation, elongation, and termination.
Recall that eukaryotic DNA is bound to proteins known as histones to form structures called nucleosomes. During initiation, the DNA is made accessible to the proteins and enzymes involved in the replication process. How does the replication machinery know where on the DNA double helix to begin? It turns out that there are specific nucleotide sequences called origins of replication at which replication begins. Certain proteins bind to the origin of replication while an enzyme called helicase unwinds and opens up the DNA helix. As the DNA opens up, Y-shaped structures called replication forks are formed (Figure 9.10). Two replication forks are formed at the origin of replication, and these get extended in both directions as replication proceeds. There are multiple origins of replication on the eukaryotic chromosome, such that replication can occur simultaneously from several places in the genome.
During elongation, an enzyme called DNA polymerase adds DNA nucleotides to the 3′ end of the template. Because DNA polymerase can only add new nucleotides at the end of a backbone, a primer sequence, which provides this starting point, is added with complementary RNA nucleotides. This primer is removed later, and the nucleotides are replaced with DNA nucleotides. One strand, which is complementary to the parental DNA strand, is synthesized continuously toward the replication fork so the polymerase can add nucleotides in this direction. This continuously synthesized strand is known as the leading strand. Because DNA polymerase can only synthesize DNA in a 5′ to 3′ direction, the other new strand is put together in short pieces called Okazaki fragments. The Okazaki fragments each require a primer made of RNA to start the synthesis. The strand with the Okazaki fragments is known as the lagging strand. As synthesis proceeds, an enzyme removes the RNA primer, which is then replaced with DNA nucleotides, and the gaps between fragments are sealed by an enzyme called DNA ligase.
The process of DNA replication can be summarized as follows:
- DNA unwinds at the origin of replication.
- New bases are added to the complementary parental strands. One new strand is made continuously, while the other strand is made in pieces.
- Primers are removed, new DNA nucleotides are put in place of the primers and the backbone is sealed by DNA ligase.
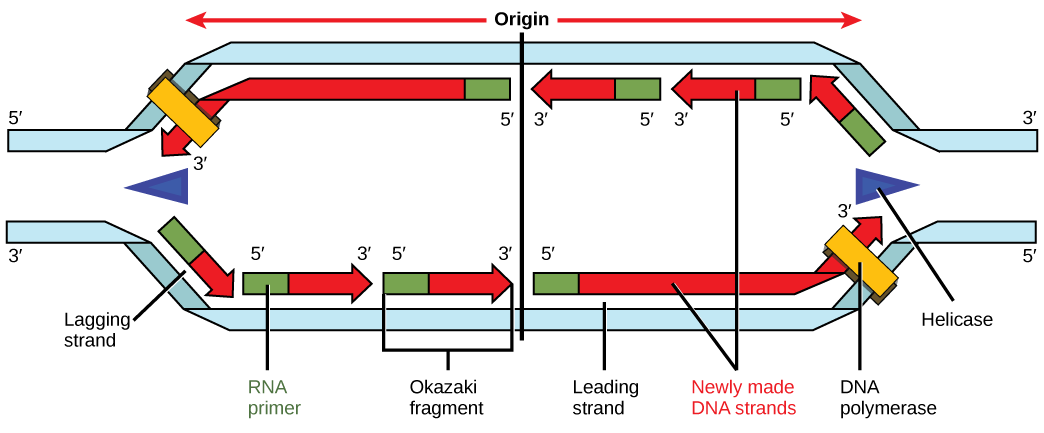
You isolate a cell strain in which the joining together of Okazaki fragments is impaired and suspect that a mutation has occurred in an enzyme found at the replication fork. Which enzyme is most likely to be mutated?
Telomere Replication
Because eukaryotic chromosomes are linear, DNA replication comes to the end of a line in eukaryotic chromosomes. As you have learned, the DNA polymerase enzyme can add nucleotides in only one direction. In the leading strand, synthesis continues until the end of the chromosome is reached; however, on the lagging strand there is no place for a primer to be made for the DNA fragment to be copied at the end of the chromosome. This presents a problem for the cell because the ends remain unpaired, and over time these ends get progressively shorter as cells continue to divide. The ends of the linear chromosomes are known as telomeres, which have repetitive sequences that do not code for a particular gene. As a consequence, it is telomeres that are shortened with each round of DNA replication instead of genes. For example, in humans, a six base-pair sequence, TTAGGG, is repeated 100 to 1000 times. The discovery of the enzyme telomerase (Figure 9.11) helped in the understanding of how chromosome ends are maintained. The telomerase attaches to the end of the chromosome, and complementary bases to the RNA template are added on the end of the DNA strand. Once the lagging strand template is sufficiently elongated, DNA polymerase can now add nucleotides that are complementary to the ends of the chromosomes. Thus, the ends of the chromosomes are replicated.
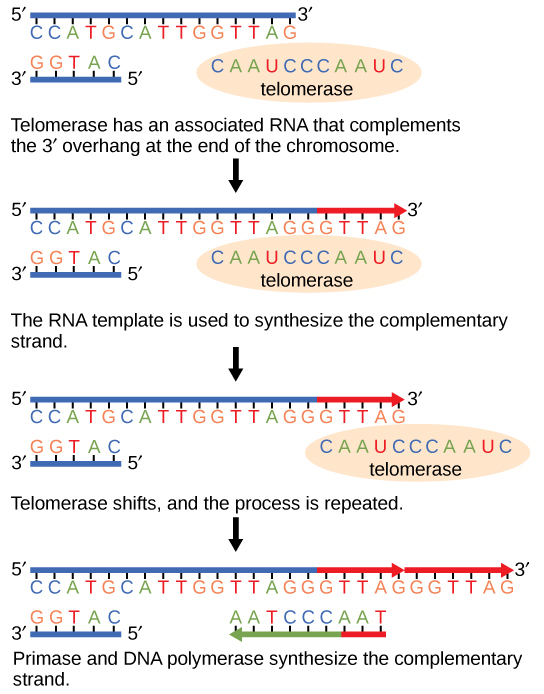
Telomerase is typically found to be active in germ cells, adult stem cells, and some cancer cells. For her discovery of telomerase and its action, Elizabeth Blackburn (Figure 9.12) received the Nobel Prize for Medicine and Physiology in 2009.

Telomerase is not active in adult somatic cells. Adult somatic cells that undergo cell division continue to have their telomeres shortened. This essentially means that telomere shortening is associated with aging. In 2010, scientists found that telomerase can reverse some age-related conditions in mice, and this may have potential in regenerative medicine.[6] Telomerase-deficient mice were used in these studies; these mice have tissue atrophy, stem-cell depletion, organ system failure, and impaired tissue injury responses. Telomerase reactivation in these mice caused extension of telomeres, reduced DNA damage, reversed neurodegeneration, and improved functioning of the testes, spleen, and intestines. Thus, telomere reactivation may have potential for treating age-related diseases in humans.
DNA Replication in Prokaryotes
Recall that the prokaryotic chromosome is a circular molecule with a less extensive coiling structure than eukaryotic chromosomes. The eukaryotic chromosome is linear and highly coiled around proteins. While there are many similarities in the DNA replication process, these structural differences necessitate some differences in the DNA replication process in these two life forms.
DNA replication has been extremely well-studied in prokaryotes, primarily because of the small size of the genome and large number of variants available. Escherichia coli has 4.6 million base pairs in a single circular chromosome, and all of it gets replicated in approximately 42 minutes, starting from a single origin of replication and proceeding around the chromosome in both directions. This means that approximately 1000 nucleotides are added per second. The process is much more rapid than in eukaryotes. Table 9.1 summarizes the differences between prokaryotic and eukaryotic replications.
| Differences between Prokaryotic and Eukaryotic Replications | ||
|---|---|---|
| Property | Prokaryotes | Eukaryotes |
| Origin of replication | Single | Multiple |
| Rate of replication | 1000 nucleotides/s | 50 to 100 nucleotides/s |
| Chromosome structure | circular | linear |
| Telomerase | Not present | Present |

Click through a tutorial on DNA replication.
DNA Repair
DNA polymerase can make mistakes while adding nucleotides. It edits the DNA by proofreading every newly added base. Incorrect bases are removed and replaced by the correct base, and then polymerization continues (Figure 9.13a). Most mistakes are corrected during replication, although when this does not happen, the mismatch repair mechanism is employed. Mismatch repair enzymes recognize the wrongly incorporated base and excise it from the DNA, replacing it with the correct base (Figure 9.13b). In yet another type of repair, nucleotide excision repair, the DNA double strand is unwound and separated, the incorrect bases are removed along with a few bases on the 5′ and 3′ end, and these are replaced by copying the template with the help of DNA polymerase (Figure 9.13c). Nucleotide excision repair is particularly important in correcting thymine dimers, which are primarily caused by ultraviolet light. In a thymine dimer, two thymine nucleotides adjacent to each other on one strand are covalently bonded to each other rather than their complementary bases. If the dimer is not removed and repaired it will lead to a mutation. Individuals with flaws in their nucleotide excision repair genes show extreme sensitivity to sunlight and develop skin cancers early in life.
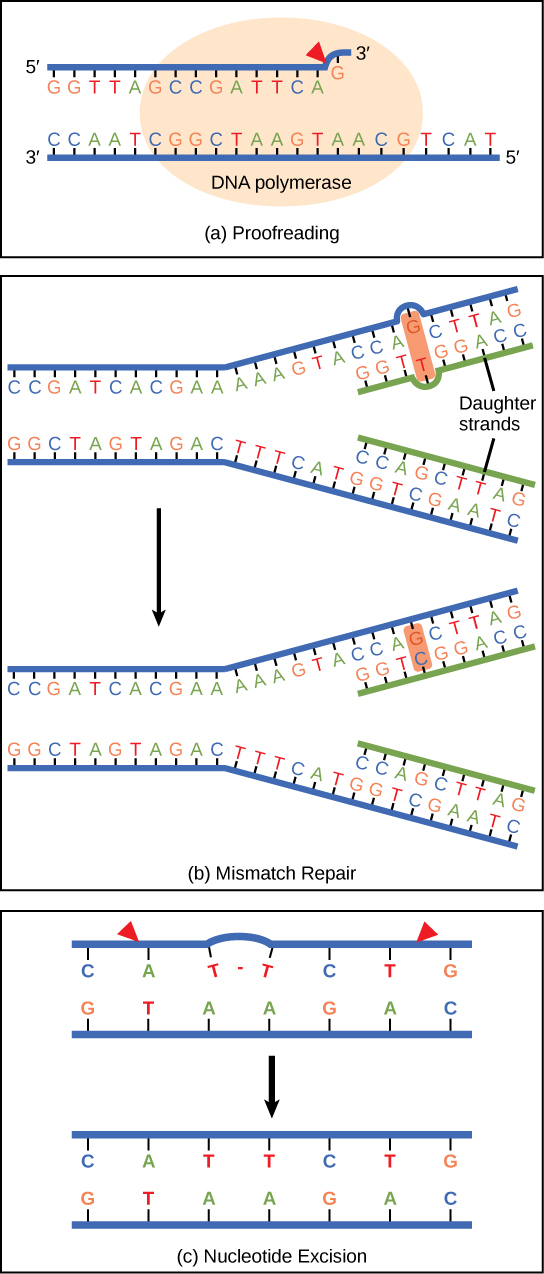
Most mistakes are corrected; if they are not, they may result in a mutation—defined as a permanent change in the DNA sequence. Mutations in repair genes may lead to serious consequences like cancer.
9.3. Transcription*
By the end of this section, you will be able to:
- Explain the central dogma
- Explain the main steps of transcription
- Describe how eukaryotic mRNA is processed
In both prokaryotes and eukaryotes, the second function of DNA (the first was replication) is to provide the information needed to construct the proteins necessary so that the cell can perform all of its functions. To do this, the DNA is “read” or transcribed into an mRNA molecule. The mRNA then provides the code to form a protein by a process called translation. Through the processes of transcription and translation, a protein is built with a specific sequence of amino acids that was originally encoded in the DNA. This module discusses the details of transcription.
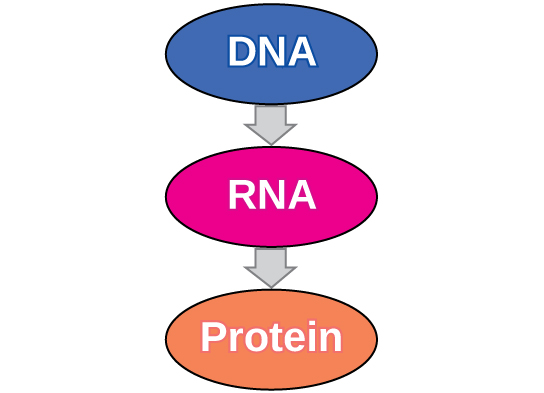
The Central Dogma: DNA Encodes RNA; RNA Encodes Protein
The flow of genetic information in cells from DNA to mRNA to protein is described by the central dogma (Figure 9.14), which states that genes specify the sequences of mRNAs, which in turn specify the sequences of proteins.
The copying of DNA to mRNA is relatively straightforward, with one nucleotide being added to the mRNA strand for every complementary nucleotide read in the DNA strand. The translation to protein is more complex because groups of three mRNA nucleotides correspond to one amino acid of the protein sequence. However, as we shall see in the next module, the translation to protein is still systematic, such that nucleotides 1 to 3 correspond to amino acid 1, nucleotides 4 to 6 correspond to amino acid 2, and so on.
Transcription: from DNA to mRNA
Both prokaryotes and eukaryotes perform fundamentally the same process of transcription, with the important difference of the membrane-bound nucleus in eukaryotes. With the genes bound in the nucleus, transcription occurs in the nucleus of the cell and the mRNA transcript must be transported to the cytoplasm. The prokaryotes, which include bacteria and archaea, lack membrane-bound nuclei and other organelles, and transcription occurs in the cytoplasm of the cell. In both prokaryotes and eukaryotes, transcription occurs in three main stages: initiation, elongation, and termination.
Initiation
Transcription requires the DNA double helix to partially unwind in the region of mRNA synthesis. The region of unwinding is called a transcription bubble. The DNA sequence onto which the proteins and enzymes involved in transcription bind to initiate the process is called a promoter. In most cases, promoters exist upstream of the genes they regulate. The specific sequence of a promoter is very important because it determines whether the corresponding gene is transcribed all of the time, some of the time, or hardly at all (Figure 9.15).
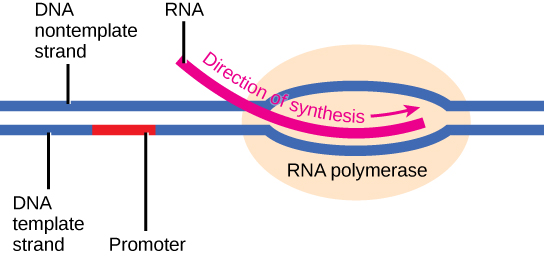
Elongation
Transcription always proceeds from one of the two DNA strands, which is called the template strand. The mRNA product is complementary to the template strand and is almost identical to the other DNA strand, called the nontemplate strand, with the exception that RNA contains a uracil (U) in place of the thymine (T) found in DNA. During elongation, an enzyme called RNA polymerase proceeds along the DNA template adding nucleotides by base pairing with the DNA template in a manner similar to DNA replication, with the difference that an RNA strand is being synthesized that does not remain bound to the DNA template. As elongation proceeds, the DNA is continuously unwound ahead of the core enzyme and rewound behind it (Figure 9.16).
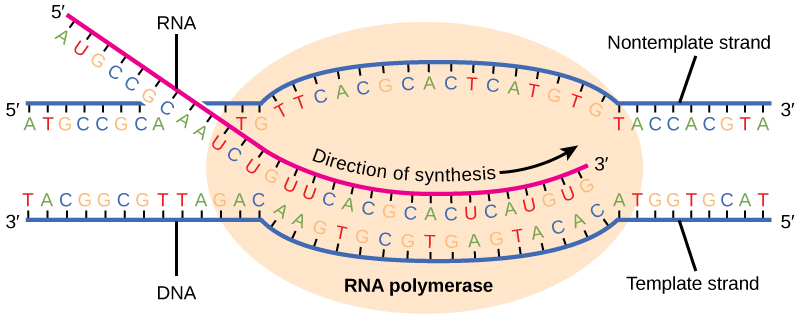
Termination
Once a gene is transcribed, the prokaryotic polymerase needs to be instructed to dissociate from the DNA template and liberate the newly made mRNA. Depending on the gene being transcribed, there are two kinds of termination signals, but both involve repeated nucleotide sequences in the DNA template that result in RNA polymerase stalling, leaving the DNA template, and freeing the mRNA transcript.
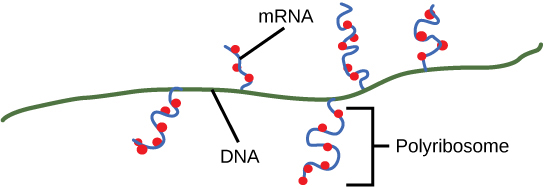
On termination, the process of transcription is complete. In a prokaryotic cell, by the time termination occurs, the transcript would already have been used to partially synthesize numerous copies of the encoded protein because these processes can occur concurrently using multiple ribosomes (polyribosomes) (Figure 9.17). In contrast, the presence of a nucleus in eukaryotic cells precludes simultaneous transcription and translation.
Eukaryotic RNA Processing
The newly transcribed eukaryotic mRNAs must undergo several processing steps before they can be transferred from the nucleus to the cytoplasm and translated into a protein. The additional steps involved in eukaryotic mRNA maturation create a molecule that is much more stable than a prokaryotic mRNA. For example, eukaryotic mRNAs last for several hours, whereas the typical prokaryotic mRNA lasts no more than five seconds.
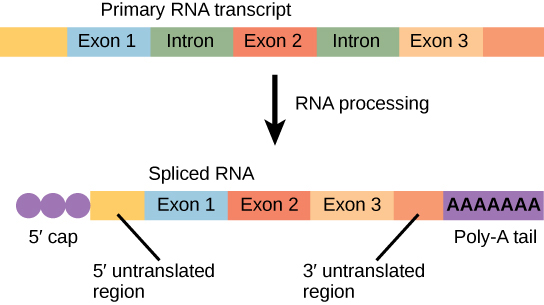
The mRNA transcript is first coated in RNA-stabilizing proteins to prevent it from degrading while it is processed and exported out of the nucleus. This occurs while the pre-mRNA still is being synthesized by adding a special nucleotide “cap” to the 5′ end of the growing transcript. In addition to preventing degradation, factors involved in protein synthesis recognize the cap to help initiate translation by ribosomes.
Once elongation is complete, an enzyme then adds a string of approximately 200 adenine residues to the 3′ end, called the poly-A tail. This modification further protects the pre-mRNA from degradation and signals to cellular factors that the transcript needs to be exported to the cytoplasm.
Eukaryotic genes are composed of protein-coding sequences called exons (ex-on signifies that they are expressed) and intervening sequences called introns (int-ron denotes their intervening role). Introns are removed from the pre-mRNA during processing. Intron sequences in mRNA do not encode functional proteins. It is essential that all of a pre-mRNA’s introns be completely and precisely removed before protein synthesis so that the exons join together to code for the correct amino acids. If the process errs by even a single nucleotide, the sequence of the rejoined exons would be shifted, and the resulting protein would be nonfunctional. The process of removing introns and reconnecting exons is called splicing (Figure 9.18). Introns are removed and degraded while the pre-mRNA is still in the nucleus.
9.4. Translation*
By the end of this section, you will be able to:
- Describe the different steps in protein synthesis
- Discuss the role of ribosomes in protein synthesis
- Describe the genetic code and how the nucleotide sequence determines the amino acid and the protein sequence
The synthesis of proteins is one of a cell’s most energy-consuming metabolic processes. In turn, proteins account for more mass than any other component of living organisms (with the exception of water), and proteins perform a wide variety of the functions of a cell. The process of translation, or protein synthesis, involves decoding an mRNA message into a polypeptide product. Amino acids are covalently strung together in lengths ranging from approximately 50 amino acids to more than 1,000.
The Protein Synthesis Machinery
In addition to the mRNA template, many other molecules contribute to the process of translation. The composition of each component may vary across species; for instance, ribosomes may consist of different numbers of ribosomal RNAs ( rRNA) and polypeptides depending on the organism. However, the general structures and functions of the protein synthesis machinery are comparable from bacteria to human cells. Translation requires the input of an mRNA template, ribosomes, tRNAs, and various enzymatic factors (Figure 9.19).
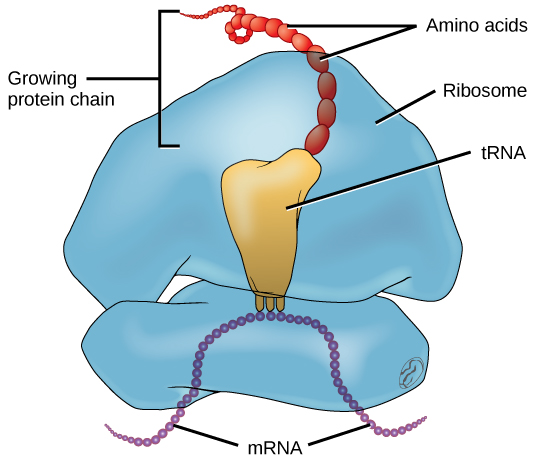
In E. coli, there are 200,000 ribosomes present in every cell at any given time. A ribosome is a complex macromolecule composed of structural and catalytic rRNAs, and many distinct polypeptides. In eukaryotes, the nucleolus is completely specialized for the synthesis and assembly of rRNAs.
Ribosomes are located in the cytoplasm in prokaryotes and in the cytoplasm and endoplasmic reticulum of eukaryotes. Ribosomes are made up of a large and a small subunit that come together for translation. The small subunit is responsible for binding the mRNA template, whereas the large subunit sequentially binds tRNAs, a type of RNA molecule that brings amino acids to the growing chain of the polypeptide. Each mRNA molecule is simultaneously translated by many ribosomes, all synthesizing protein in the same direction.
Depending on the species, 40 to 60 types of tRNA exist in the cytoplasm. Serving as adaptors, specific tRNAs bind to sequences on the mRNA template and add the corresponding amino acid to the polypeptide chain. Therefore, tRNAs are the molecules that actually “translate” the language of RNA into the language of proteins. For each tRNA to function, it must have its specific amino acid bonded to it. In the process of tRNA “charging,” each tRNA molecule is bonded to its correct amino acid.
The Genetic Code
To summarize what we know to this point, the cellular process of transcription generates messenger RNA (mRNA), a mobile molecular copy of one or more genes with an alphabet of A, C, G, and uracil (U). Translation of the mRNA template converts nucleotide-based genetic information into a protein product. Protein sequences consist of 20 commonly occurring amino acids; therefore, it can be said that the protein alphabet consists of 20 letters. Each amino acid is defined by a three-nucleotide sequence called the triplet codon. The relationship between a nucleotide codon and its corresponding amino acid is called the genetic code.
Given the different numbers of “letters” in the mRNA and protein “alphabets,” combinations of nucleotides corresponded to single amino acids. Using a three-nucleotide code means that there are a total of 64 (4 × 4 × 4) possible combinations; therefore, a given amino acid is encoded by more than one nucleotide triplet (Figure 9.20).
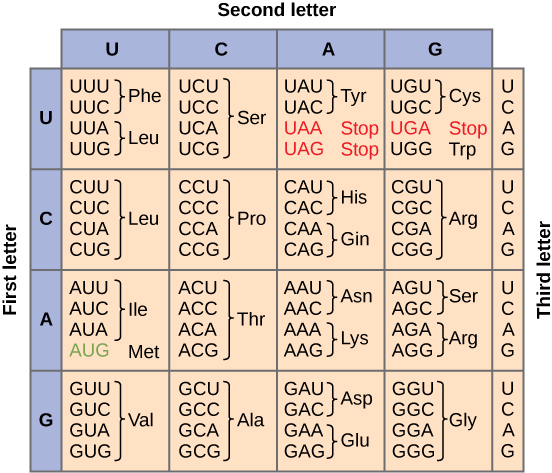
Three of the 64 codons terminate protein synthesis and release the polypeptide from the translation machinery. These triplets are called stop codons. Another codon, AUG, also has a special function. In addition to specifying the amino acid methionine, it also serves as the start codon to initiate translation. The reading frame for translation is set by the AUG start codon near the 5′ end of the mRNA. The genetic code is universal. With a few exceptions, virtually all species use the same genetic code for protein synthesis, which is powerful evidence that all life on Earth shares a common origin.
The Mechanism of Protein Synthesis
Just as with mRNA synthesis, protein synthesis can be divided into three phases: initiation, elongation, and termination. The process of translation is similar in prokaryotes and eukaryotes. Here we will explore how translation occurs in E. coli, a representative prokaryote, and specify any differences between prokaryotic and eukaryotic translation.
Protein synthesis begins with the formation of an initiation complex. In E. coli, this complex involves the small ribosome subunit, the mRNA template, three initiation factors, and a special initiator tRNA. The initiator tRNA interacts with the AUG start codon, and links to a special form of the amino acid methionine that is typically removed from the polypeptide after translation is complete.
In prokaryotes and eukaryotes, the basics of polypeptide elongation are the same, so we will review elongation from the perspective of E. coli. The large ribosomal subunit of E. coli consists of three compartments: the A site binds incoming charged tRNAs (tRNAs with their attached specific amino acids). The P site binds charged tRNAs carrying amino acids that have formed bonds with the growing polypeptide chain but have not yet dissociated from their corresponding tRNA. The E site releases dissociated tRNAs so they can be recharged with free amino acids. The ribosome shifts one codon at a time, catalyzing each process that occurs in the three sites. With each step, a charged tRNA enters the complex, the polypeptide becomes one amino acid longer, and an uncharged tRNA departs. The energy for each bond between amino acids is derived from GTP, a molecule similar to ATP (Figure 9.21). Amazingly, the E. coli translation apparatus takes only 0.05 seconds to add each amino acid, meaning that a 200-amino acid polypeptide could be translated in just 10 seconds.
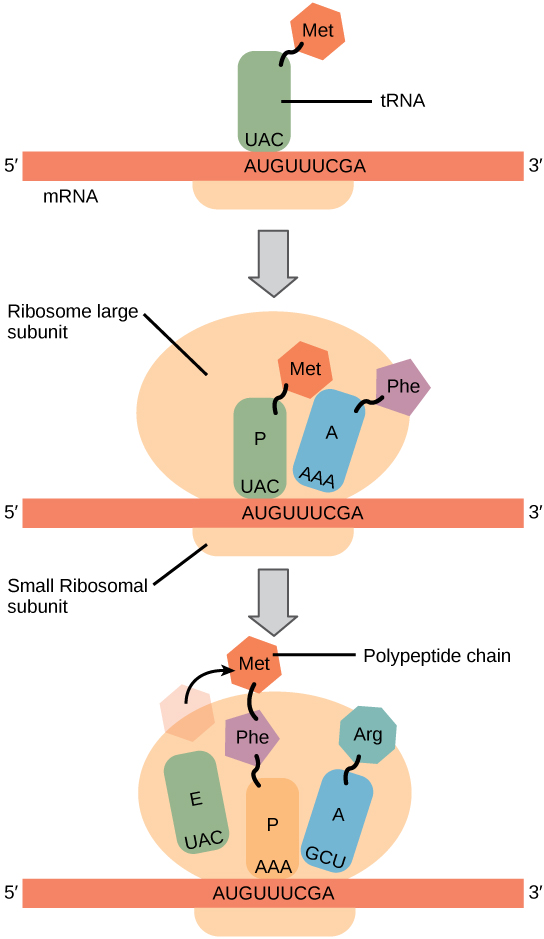
Termination of translation occurs when a stop codon (UAA, UAG, or UGA) is encountered. When the ribosome encounters the stop codon, the growing polypeptide is released and the ribosome subunits dissociate and leave the mRNA. After many ribosomes have completed translation, the mRNA is degraded so the nucleotides can be reused in another transcription reaction.

Transcribe a gene and translate it to protein using complementary pairing and the genetic code at this site.
9.5. How Genes Are Regulated*
By the end of this section, you will be able to:
- Discuss why every cell does not express all of its genes
- Describe how prokaryotic gene expression occurs at the transcriptional level
- Understand that eukaryotic gene expression occurs at the epigenetic, transcriptional, post-transcriptional, translational, and post-translational levels
For a cell to function properly, necessary proteins must be synthesized at the proper time. All organisms and cells control or regulate the transcription and translation of their DNA into protein. The process of turning on a gene to produce RNA and protein is called gene expression. Whether in a simple unicellular organism or in a complex multicellular organism, each cell controls when and how its genes are expressed. For this to occur, there must be a mechanism to control when a gene is expressed to make RNA and protein, how much of the protein is made, and when it is time to stop making that protein because it is no longer needed.
Cells in multicellular organisms are specialized; cells in different tissues look very different and perform different functions. For example, a muscle cell is very different from a liver cell, which is very different from a skin cell. These differences are a consequence of the expression of different sets of genes in each of these cells. All cells have certain basic functions they must perform for themselves, such as converting the energy in sugar molecules into energy in ATP. Each cell also has many genes that are not expressed, and expresses many that are not expressed by other cells, such that it can carry out its specialized functions. In addition, cells will turn on or off certain genes at different times in response to changes in the environment or at different times during the development of the organism. Unicellular organisms, both eukaryotic and prokaryotic, also turn on and off genes in response to the demands of their environment so that they can respond to special conditions.
The control of gene expression is extremely complex. Malfunctions in this process are detrimental to the cell and can lead to the development of many diseases, including cancer.
Prokaryotic versus Eukaryotic Gene Expression
To understand how gene expression is regulated, we must first understand how a gene becomes a functional protein in a cell. The process occurs in both prokaryotic and eukaryotic cells, just in slightly different fashions.
Because prokaryotic organisms lack a cell nucleus, the processes of transcription and translation occur almost simultaneously. When the protein is no longer needed, transcription stops. As a result, the primary method to control what type and how much protein is expressed in a prokaryotic cell is through the regulation of DNA transcription into RNA. All the subsequent steps happen automatically. When more protein is required, more transcription occurs. Therefore, in prokaryotic cells, the control of gene expression is almost entirely at the transcriptional level.
The first example of such control was discovered using E. coli in the 1950s and 1960s by French researchers and is called the lac operon. The lac operon is a stretch of DNA with three adjacent genes that code for proteins that participate in the absorption and metabolism of lactose, a food source for E. coli. When lactose is not present in the bacterium’s environment, the lac genes are transcribed in small amounts. When lactose is present, the genes are transcribed and the bacterium is able to use the lactose as a food source. The operon also contains a promoter sequence to which the RNA polymerase binds to begin transcription; between the promoter and the three genes is a region called the operator. When there is no lactose present, a protein known as a repressor binds to the operator and prevents RNA polymerase from binding to the promoter, except in rare cases. Thus very little of the protein products of the three genes is made. When lactose is present, an end product of lactose metabolism binds to the repressor protein and prevents it from binding to the operator. This allows RNA polymerase to bind to the promoter and freely transcribe the three genes, allowing the organism to metabolize the lactose.
Eukaryotic cells, in contrast, have intracellular organelles and are much more complex. Recall that in eukaryotic cells, the DNA is contained inside the cell’s nucleus and it is transcribed into mRNA there. The newly synthesized mRNA is then transported out of the nucleus into the cytoplasm, where ribosomes translate the mRNA into protein. The processes of transcription and translation are physically separated by the nuclear membrane; transcription occurs only within the nucleus, and translation only occurs outside the nucleus in the cytoplasm. The regulation of gene expression can occur at all stages of the process (Figure 9.22). Regulation may occur when the DNA is uncoiled and loosened from nucleosomes to bind transcription factors ( epigenetic level), when the RNA is transcribed (transcriptional level), when RNA is processed and exported to the cytoplasm after it is transcribed ( post-transcriptional level), when the RNA is translated into protein (translational level), or after the protein has been made ( post-translational level).
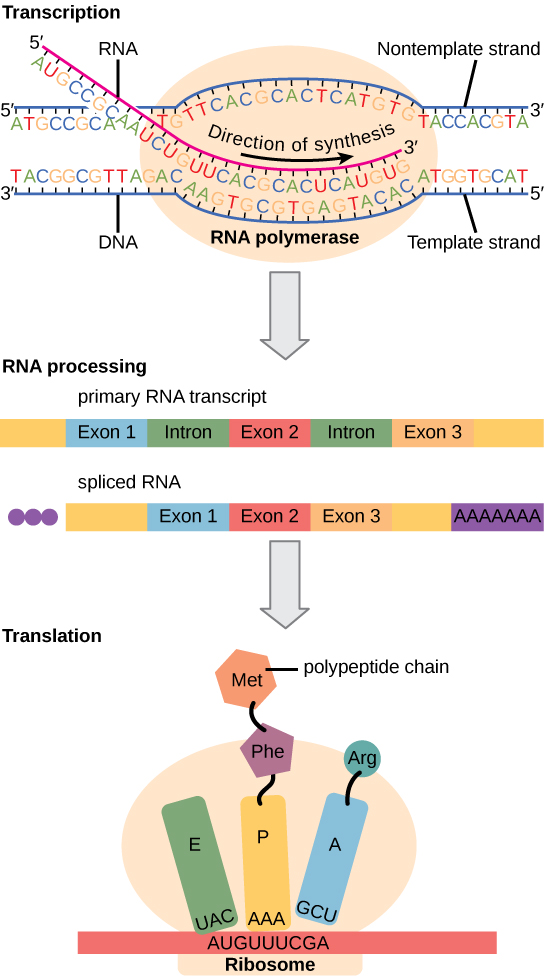
The differences in the regulation of gene expression between prokaryotes and eukaryotes are summarized in Table 9.2.
| Differences in the Regulation of Gene Expression of Prokaryotic and Eukaryotic Organisms | |
|---|---|
| Prokaryotic organisms | Eukaryotic organisms |
| Lack nucleus | Contain nucleus |
| RNA transcription and protein translation occur almost simultaneously |
|
| Gene expression is regulated primarily at the transcriptional level | Gene expression is regulated at many levels (epigenetic, transcriptional, post-transcriptional, translational, and post-translational) |
In the 1970s, genes were first observed that exhibited alternative RNA splicing. Alternative RNA splicing is a mechanism that allows different protein products to be produced from one gene when different combinations of introns (and sometimes exons) are removed from the transcript (Figure 9.23). This alternative splicing can be haphazard, but more often it is controlled and acts as a mechanism of gene regulation, with the frequency of different splicing alternatives controlled by the cell as a way to control the production of different protein products in different cells, or at different stages of development. Alternative splicing is now understood to be a common mechanism of gene regulation in eukaryotes; according to one estimate, 70% of genes in humans are expressed as multiple proteins through alternative splicing.
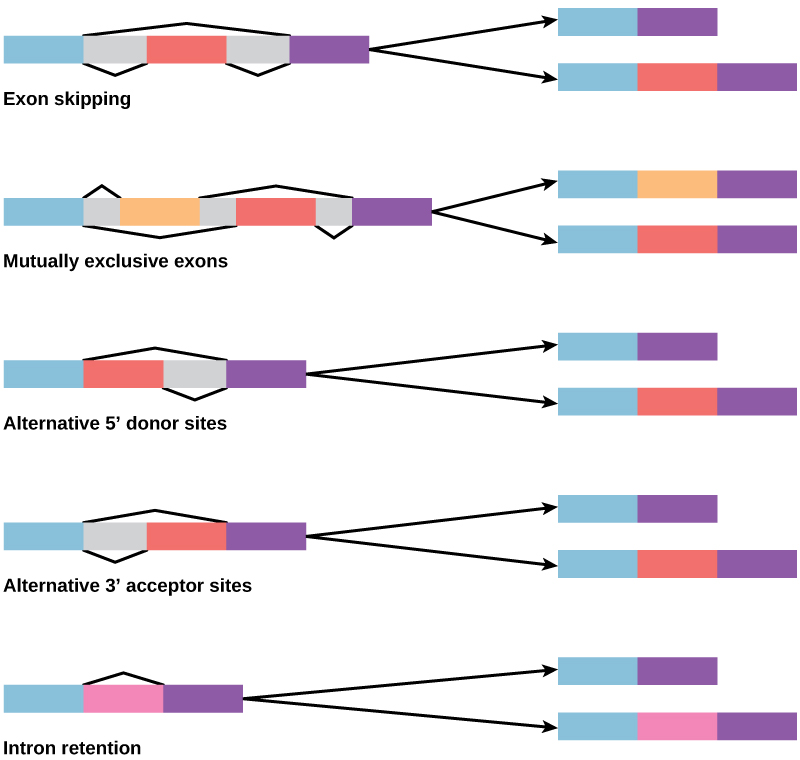
How could alternative splicing evolve? Introns have a beginning and ending recognition sequence, and it is easy to imagine the failure of the splicing mechanism to identify the end of an intron and find the end of the next intron, thus removing two introns and the intervening exon. In fact, there are mechanisms in place to prevent such exon skipping, but mutations are likely to lead to their failure. Such “mistakes” would more than likely produce a nonfunctional protein. Indeed, the cause of many genetic diseases is alternative splicing rather than mutations in a sequence. However, alternative splicing would create a protein variant without the loss of the original protein, opening up possibilities for adaptation of the new variant to new functions. Gene duplication has played an important role in the evolution of new functions in a similar way—by providing genes that may evolve without eliminating the original functional protein.
Glossary
- alternative RNA splicing
- a post-transcriptional gene regulation mechanism in eukaryotes in which multiple protein products are produced by a single gene through alternative splicing combinations of the RNA transcript
- codon
- three consecutive nucleotides in mRNA that specify the addition of a specific amino acid or the release of a polypeptide chain during translation
- DNA ligase
- the enzyme that catalyzes the joining of DNA fragments together
- DNA polymerase
- an enzyme that synthesizes a new strand of DNA complementary to a template strand
- deoxyribose
- a five-carbon sugar molecule with a hydrogen atom rather than a hydroxyl group in the 2′ position; the sugar component of DNA nucleotides
- double helix
- the molecular shape of DNA in which two strands of nucleotides wind around each other in a spiral shape
- epigenetic
- describing non-genetic regulatory factors, such as changes in modifications to histone proteins and DNA that control accessibility to genes in chromosomes
- exon
- a sequence present in protein-coding mRNA after completion of pre-mRNA splicing
- gene expression
- processes that control whether a gene is expressed
- genetic code
- the amino acids that correspond to three-nucleotide codons of mRNA
- helicase
- an enzyme that helps to open up the DNA helix during DNA replication by breaking the hydrogen bonds
- intron
- non–protein-coding intervening sequences that are spliced from mRNA during processing
- lagging strand
- during replication of the 3′ to 5′ strand, the strand that is replicated in short fragments and away from the replication fork
- leading strand
- the strand that is synthesized continuously in the 5′ to 3′ direction that is synthesized in the direction of the replication fork
- mRNA
- messenger RNA; a form of RNA that carries the nucleotide sequence code for a protein sequence that is translated into a polypeptide sequence
- mismatch repair
- a form of DNA repair in which non-complementary nucleotides are recognized, excised, and replaced with correct nucleotides
- mutation
- a permanent variation in the nucleotide sequence of a genome
- nitrogenous base
- a nitrogen-containing molecule that acts as a base; often referring to one of the purine or pyrimidine components of nucleic acids
- nontemplate strand
- the strand of DNA that is not used to transcribe mRNA; this strand is identical to the mRNA except that T nucleotides in the DNA are replaced by U nucleotides in the mRNA
- nucleotide excision repair
- a form of DNA repair in which the DNA molecule is unwound and separated in the region of the nucleotide damage, the damaged nucleotides are removed and replaced with new nucleotides using the complementary strand, and the DNA strand is resealed and allowed to rejoin its complement
- Okazaki fragments
- the DNA fragments that are synthesized in short stretches on the lagging strand
- phosphate group
- a molecular group consisting of a central phosphorus atom bound to four oxygen atoms
- post-transcriptional
- control of gene expression after the RNA molecule has been created but before it is translated into protein
- post-translational
- control of gene expression after a protein has been created
- primer
- a short stretch of RNA nucleotides that is required to initiate replication and allow DNA polymerase to bind and begin replication
- promoter
- a sequence on DNA to which RNA polymerase and associated factors bind and initiate transcription
- RNA polymerase
- an enzyme that synthesizes an RNA strand from a DNA template strand
- rRNA
- ribosomal RNA; molecules of RNA that combine to form part of the ribosome
- replication fork
- the Y-shaped structure formed during the initiation of replication
- semiconservative replication
- the method used to replicate DNA in which the double-stranded molecule is separated and each strand acts as a template for a new strand to be synthesized, so the resulting DNA molecules are composed of one new strand of nucleotides and one old strand of nucleotides
- splicing
- the process of removing introns and reconnecting exons in a pre-mRNA
- start codon
- the AUG (or, rarely GUG) on an mRNA from which translation begins; always specifies methionine
- stop codon
- one of the three mRNA codons that specifies termination of translation
- tRNA
- transfer RNA; an RNA molecule that contains a specific three-nucleotide anticodon sequence to pair with the mRNA codon and also binds to a specific amino acid
- telomerase
- an enzyme that contains a catalytic part and an inbuilt RNA template; it functions to maintain telomeres at chromosome ends
- telomere
- the DNA at the end of linear chromosomes
- template strand
- the strand of DNA that specifies the complementary mRNA molecule
- transcription bubble
- the region of locally unwound DNA that allows for transcription of mRNA
<!–CNX: Start Area: “Sections Summary”–>
The model of the double-helix structure of DNA was proposed by Watson and Crick. The DNA molecule is a polymer of nucleotides. Each nucleotide is composed of a nitrogenous base, a five-carbon sugar (deoxyribose), and a phosphate group. There are four nitrogenous bases in DNA, two purines (adenine and guanine) and two pyrimidines (cytosine and thymine). A DNA molecule is composed of two strands. Each strand is composed of nucleotides bonded together covalently between the phosphate group of one and the deoxyribose sugar of the next. From this backbone extend the bases. The bases of one strand bond to the bases of the second strand with hydrogen bonds. Adenine always bonds with thymine, and cytosine always bonds with guanine. The bonding causes the two strands to spiral around each other in a shape called a double helix. Ribonucleic acid (RNA) is a second nucleic acid found in cells. RNA is a single-stranded polymer of nucleotides. It also differs from DNA in that it contains the sugar ribose, rather than deoxyribose, and the nucleotide uracil rather than thymine. Various RNA molecules function in the process of forming proteins from the genetic code in DNA.
Prokaryotes contain a single, double-stranded circular chromosome. Eukaryotes contain double-stranded linear DNA molecules packaged into chromosomes. The DNA helix is wrapped around proteins to form nucleosomes. The protein coils are further coiled, and during mitosis and meiosis, the chromosomes become even more greatly coiled to facilitate their movement. Chromosomes have two distinct regions which can be distinguished by staining, reflecting different degrees of packaging and determined by whether the DNA in a region is being expressed (euchromatin) or not (heterochromatin).
DNA replicates by a semi-conservative method in which each of the two parental DNA strands act as a template for new DNA to be synthesized. After replication, each DNA has one parental or “old” strand, and one daughter or “new” strand.
Replication in eukaryotes starts at multiple origins of replication, while replication in prokaryotes starts from a single origin of replication. The DNA is opened with enzymes, resulting in the formation of the replication fork. Primase synthesizes an RNA primer to initiate synthesis by DNA polymerase, which can add nucleotides in only one direction. One strand is synthesized continuously in the direction of the replication fork; this is called the leading strand. The other strand is synthesized in a direction away from the replication fork, in short stretches of DNA known as Okazaki fragments. This strand is known as the lagging strand. Once replication is completed, the RNA primers are replaced by DNA nucleotides and the DNA is sealed with DNA ligase.
The ends of eukaryotic chromosomes pose a problem, as polymerase is unable to extend them without a primer. Telomerase, an enzyme with an inbuilt RNA template, extends the ends by copying the RNA template and extending one end of the chromosome. DNA polymerase can then extend the DNA using the primer. In this way, the ends of the chromosomes are protected. Cells have mechanisms for repairing DNA when it becomes damaged or errors are made in replication. These mechanisms include mismatch repair to replace nucleotides that are paired with a non-complementary base and nucleotide excision repair, which removes bases that are damaged such as thymine dimers.
In prokaryotes, mRNA synthesis is initiated at a promoter sequence on the DNA template. Elongation synthesizes new mRNA. Termination liberates the mRNA and occurs by mechanisms that stall the RNA polymerase and cause it to fall off the DNA template. Newly transcribed eukaryotic mRNAs are modified with a cap and a poly-A tail. These structures protect the mature mRNA from degradation and help export it from the nucleus. Eukaryotic mRNAs also undergo splicing, in which introns are removed and exons are reconnected with single-nucleotide accuracy. Only finished mRNAs are exported from the nucleus to the cytoplasm.
The central dogma describes the flow of genetic information in the cell from genes to mRNA to proteins. Genes are used to make mRNA by the process of transcription; mRNA is used to synthesize proteins by the process of translation. The genetic code is the correspondence between the three-nucleotide mRNA codon and an amino acid. The genetic code is “translated” by the tRNA molecules, which associate a specific codon with a specific amino acid. The genetic code is degenerate because 64 triplet codons in mRNA specify only 20 amino acids and three stop codons. This means that more than one codon corresponds to an amino acid. Almost every species on the planet uses the same genetic code.
The players in translation include the mRNA template, ribosomes, tRNAs, and various enzymatic factors. The small ribosomal subunit binds to the mRNA template. Translation begins at the initiating AUG on the mRNA. The formation of bonds occurs between sequential amino acids specified by the mRNA template according to the genetic code. The ribosome accepts charged tRNAs, and as it steps along the mRNA, it catalyzes bonding between the new amino acid and the end of the growing polypeptide. The entire mRNA is translated in three-nucleotide “steps” of the ribosome. When a stop codon is encountered, a release factor binds and dissociates the components and frees the new protein.
While all somatic cells within an organism contain the same DNA, not all cells within that organism express the same proteins. Prokaryotic organisms express the entire DNA they encode in every cell, but not necessarily all at the same time. Proteins are expressed only when they are needed. Eukaryotic organisms express a subset of the DNA that is encoded in any given cell. In each cell type, the type and amount of protein is regulated by controlling gene expression. To express a protein, the DNA is first transcribed into RNA, which is then translated into proteins. In prokaryotic cells, these processes occur almost simultaneously. In eukaryotic cells, transcription occurs in the nucleus and is separate from the translation that occurs in the cytoplasm. Gene expression in prokaryotes is regulated only at the transcriptional level, whereas in eukaryotic cells, gene expression is regulated at the epigenetic, transcriptional, post-transcriptional, translational, and post-translational levels.
<!–CNX: Start Area: “Art Connections”–>
Figure 9.10 You isolate a cell strain in which the joining together of Okazaki fragments is impaired and suspect that a mutation has occurred in an enzyme found at the replication fork. Which enzyme is most likely to be mutated?
Figure 9.10 Ligase, as this enzyme joins together Okazaki fragments.
<!–CNX: Start Area: “Multiple Choice”–>
Which of the following does cytosine pair with?
- guanine
- thymine
- adenine
- a pyrimidine
A
- single-stranded circular; single-stranded linear
- single-stranded linear; single-stranded circular
- double-stranded circular; double-stranded linear
- double-stranded linear; double-stranded circular
C
- conservative
- semiconservative
- dispersive
- none of the above
B
- mismatch repair
- DNA polymerase proofreading
- nucleotide excision repair
- thymine dimers
B
- a specific sequence of DNA nucleotides
- a specific sequence of RNA nucleotides
- a protein that binds to DNA
- an enzyme that synthesizes RNA
A
- exons
- caps
- poly-A tails
- introns
D
- cytoplasm
- nucleus
- nucleolus
- endoplasmic reticulum
C
How long would the peptide be that is translated from this MRNA sequence: 5′-AUGGGCUACCGA-3′?
- 0
- 2
- 3
- 4
D
- only the transcriptional level
- epigenetic and transcriptional levels
- epigenetic, transcriptional, and translational levels
- epigenetic, transcriptional, post-transcriptional, translational, and post-translational levels
D
- regulation of gene expression after transcription
- regulation of gene expression after translation
- control of epigenetic activation
- period between transcription and translation
B
<!–CNX: Start Area: “Free Response”–>
Describe the organization of the eukaryotic chromosome.
The DNA is wound around proteins called histones. The histones then stack together in a compact form that creates a fiber that is 30-nm thick. The fiber is further coiled for greater compactness. During metaphase of mitosis, the chromosome is at its most compact to facilitate chromosome movement. During interphase, there are denser areas of chromatin, called heterochromatin, that contain DNA that is not expressed, and less dense euchromatin that contains DNA that is expressed.
A single strand of DNA is a polymer of nucleic acids joined covalently between the phosphate group of one and the deoxyribose sugar of the next to for a “backbone” from which the nitrogenous bases stick out. In its natural state, DNA has two strands wound around each other in a double helix. The bases on each strand are bonded to each other with hydrogen bonds. Only specific bases bond with each other; adenine bonds with thymine, and cytosine bonds with guanine.
Telomerase has an inbuilt RNA template that extends the 3′ end, so a primer is synthesized and extended. Thus, the ends are protected.
The mRNA would be: 5′-AUGGCCGGUUAUUAAGCA-3′. The protein would be: MAGY. Even though there are six codons, the fifth codon corresponds to a stop, so the sixth codon would not be translated.
The cell controls which protein is expressed, and to what level that protein is expressed, in the cell. Prokaryotic cells alter the transcription rate to turn genes on or off. This method will increase or decrease protein levels in response to what is needed by the cell. Eukaryotic cells change the accessibility (epigenetic), transcription, or translation of a gene. This will alter the amount of RNA, and the lifespan of the RNA, to alter the amount of protein that exists. Eukaryotic cells also change the protein’s translation to increase or decrease its overall levels. Eukaryotic organisms are much more complex and can manipulate protein levels by changing many stages in the process.
<!–CNX: Start Area: “Art Connections”–>
Figure 9.10 Ligase, as this enzyme joins together Okazaki fragments.
<!–CNX: Start Area: “Multiple Choice”–>
A
C
B
B
A
D
C
D
B
<!–CNX: Start Area: “Free Response”–>
The DNA is wound around proteins called histones. The histones then stack together in a compact form that creates a fiber that is 30-nm thick. The fiber is further coiled for greater compactness. During metaphase of mitosis, the chromosome is at its most compact to facilitate chromosome movement. During interphase, there are denser areas of chromatin, called heterochromatin, that contain DNA that is not expressed, and less dense euchromatin that contains DNA that is expressed.
A single strand of DNA is a polymer of nucleic acids joined covalently between the phosphate group of one and the deoxyribose sugar of the next to for a “backbone” from which the nitrogenous bases stick out. In its natural state, DNA has two strands wound around each other in a double helix. The bases on each strand are bonded to each other with hydrogen bonds. Only specific bases bond with each other; adenine bonds with thymine, and cytosine bonds with guanine.
Telomerase has an inbuilt RNA template that extends the 3′ end, so a primer is synthesized and extended. Thus, the ends are protected.
The mRNA would be: 5′-AUGGCCGGUUAUUAAGCA-3′. The protein would be: MAGY. Even though there are six codons, the fifth codon corresponds to a stop, so the sixth codon would not be translated.
The cell controls which protein is expressed, and to what level that protein is expressed, in the cell. Prokaryotic cells alter the transcription rate to turn genes on or off. This method will increase or decrease protein levels in response to what is needed by the cell. Eukaryotic cells change the accessibility (epigenetic), transcription, or translation of a gene. This will alter the amount of RNA, and the lifespan of the RNA, to alter the amount of protein that exists. Eukaryotic cells also change the protein’s translation to increase or decrease its overall levels. Eukaryotic organisms are much more complex and can manipulate protein levels by changing many stages in the process.